Meaning Of Orphan Drug Designation

According to the us food and drug administration fda an orphan drug is defined as one intended for the treatment prevention or diagnosis of a rare disease or condition which is one that affects less than 200 000 persons in the united states which equates to roughly 6 in 10 000 people in the european union eu the european medicines agency ema defined a drug as orphan.
Meaning of orphan drug designation. Under the orphan drug act drug companies can apply for orphan drug designation odd and if granted the drug will have a status which gives companies exclusive marketing and development rights along with other benefits to recover the costs of researching and developing the drug. Each designation request must stand on its. Orphan drug designation in the european union provides a 10 year period of post approval marketing exclusivity and other financial incentives for new drugs that offer significant therapeutic advantage over other approved treatments of rare conditions conditions affecting no more than 5 in 10 000 persons.
A drug or biological product that treats a rare condition or disease. Orphan drug designation simply determines a company s qualification to receive the incentives provided under the orphan drug act. Orphan drug designation explained the fda s office of orphan products development is tasked with advancing the evaluation and development of drugs biologics devices or medical foods that could.
Final thoughts obtaining orphan designation is newsworthy and is an important milestone that provides significant drug development business and financial incentives. Sponsors can receive orphan drug designation for the use of a drug in an orphan subset of a non rare disease or condition when they can explain based on a characteristic or feature of the drug e. What the orphan drug designation accomplishes for developers.
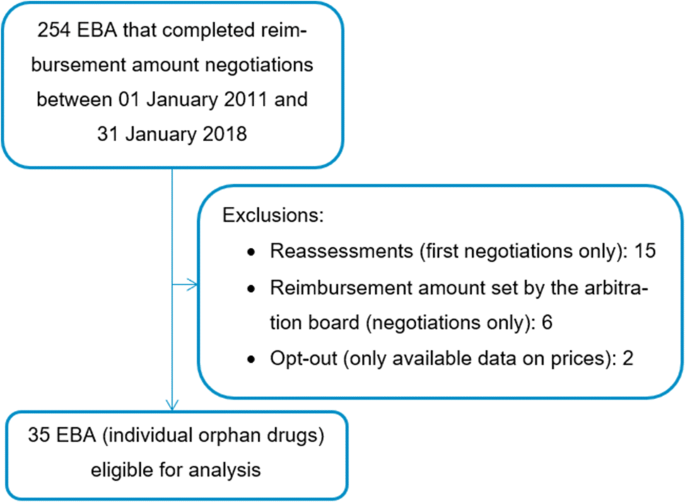
A sponsor seeking orphan designation for a drug must submit a request for designation to oopd with the information required in 21 cfr 316 20 and 316 21.






:max_bytes(150000):strip_icc()/GettyImages-1135301768-95d6d47444fc44258ca4338f4b90f925.jpg)